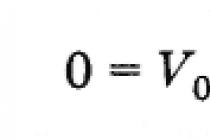Absolute temperature zero corresponds to 273.15 degrees Celsius below zero, 459.67 below zero Fahrenheit. For the Kelvin temperature scale, this temperature itself is the zero mark.
The essence of absolute zero temperature
The concept of absolute zero comes from the very essence of temperature. Any body that releases into the external environment during. At the same time, body temperature decreases, i.e. less energy remains. Theoretically, this process can continue until the amount of energy reaches such a minimum that the body can no longer give it away.
A distant harbinger of such an idea can already be found in M.V. Lomonosov. The great Russian scientist explained heat by “rotary” movement. Consequently, the maximum degree of cooling is a complete stop of such movement.
According to modern concepts, absolute zero temperature is at which molecules have the lowest possible energy level. With less energy, i.e. at a lower temperature, no physical body can exist.
Theory and practice
Absolute zero temperature is a theoretical concept; it is impossible to achieve it in practice, in principle, even in scientific laboratories with the most sophisticated equipment. But scientists manage to cool the substance to very low temperatures, which are close to absolute zero.
At such temperatures, substances acquire amazing properties that they cannot have under ordinary circumstances. Mercury, which is called "living silver" because it is in a state close to liquid, becomes solid at this temperature - to the point that it can be used to drive nails. Some metals become brittle, like glass. Rubber becomes just as hard. If you hit a rubber object with a hammer at a temperature close to absolute zero, it will break like glass.
This change in properties is also associated with the nature of heat. The higher the temperature of the physical body, the more intense and chaotic the molecules move. As the temperature decreases, the movement becomes less intense and the structure becomes more orderly. So a gas becomes a liquid, and a liquid becomes a solid. The ultimate level of order is the crystal structure. At ultra-low temperatures, even substances that normally remain amorphous, such as rubber, acquire it.
Interesting phenomena also occur with metals. The atoms of the crystal lattice vibrate with less amplitude, electron scattering decreases, and therefore electrical resistance decreases. The metal acquires superconductivity, the practical application of which seems very tempting, although difficult to achieve.
The limiting temperature at which the volume of an ideal gas becomes equal to zero is taken as absolute zero temperature. However, the volume of real gases at absolute zero temperature cannot vanish. Does this temperature limit make sense then?
The limiting temperature, the existence of which follows from the Gay-Lussac law, makes sense, since it is practically possible to bring the properties of a real gas closer to the properties of an ideal one. To do this, you need to take an increasingly rarefied gas, so that its density tends to zero. Indeed, as the temperature decreases, the volume of such a gas will tend to the limit, close to zero.
Let's find the value of absolute zero on the Celsius scale. Equating volume VV formula (3.6.4) zero and taking into account that

Hence the absolute zero temperature is

* More accurate absolute zero value: -273.15 °C.
This is the extreme, lowest temperature in nature, that “greatest or last degree of cold”, the existence of which Lomonosov predicted.
Kelvin scale

Kelvin William (Thomson W.) (1824-1907) - an outstanding English physicist, one of the founders of thermodynamics and the molecular kinetic theory of gases.
Kelvin introduced the absolute temperature scale and gave one of the formulations of the second law of thermodynamics in the form of the impossibility of completely converting heat into work. He calculated the size of molecules based on measuring the surface energy of the liquid. In connection with the laying of the transatlantic telegraph cable, Kelvin developed the theory of electromagnetic oscillations and derived a formula for the period of free oscillations in a circuit. For his scientific achievements, W. Thomson received the title of Lord Kelvin.
The English scientist W. Kelvin introduced the absolute temperature scale. Zero temperature on the Kelvin scale corresponds to absolute zero, and the unit of temperature on this scale is equal to a degree on the Celsius scale, so absolute temperature T is related to temperature on the Celsius scale by the formula
![]() (3.7.6)
(3.7.6)
Figure 3.11 shows the absolute scale and the Celsius scale for comparison.

The SI unit of absolute temperature is called the kelvin (abbreviated K). Therefore, one degree on the Celsius scale is equal to one degree on the Kelvin scale: 1 °C = 1 K.
Thus, absolute temperature, according to the definition given by formula (3.7.6), is a derived quantity that depends on the Celsius temperature and on the experimentally determined value of a. However, it is of fundamental importance.
From the point of view of molecular kinetic theory, absolute temperature is related to the average kinetic energy of the chaotic movement of atoms or molecules. At T = O K the thermal movement of molecules stops. This will be discussed in more detail in Chapter 4.
Dependence of volume on absolute temperature
Using the Kelvin scale, Gay-Lussac's law (3.6.4) can be written in a simpler form. Because
![]() (3.7.7)
(3.7.7)
The volume of a gas of a given mass at constant pressure is directly proportional to the absolute temperature.
It follows that the ratio of volumes of gas of the same mass in different states at the same pressure is equal to the ratio of absolute temperatures:
 (3.7.8)
(3.7.8)
There is a minimum possible temperature at which the volume (and pressure) of an ideal gas vanishes. This is absolute zero temperature:-273 °C. It is convenient to count the temperature from absolute zero. This is how the absolute temperature scale is constructed.
Any physical body, including all objects in the Universe, has a minimum temperature or its limit. The starting point of any temperature scale is considered to be the value of absolute zero temperature. But this is only in theory. The chaotic movement of atoms and molecules, which give up their energy at this time, has not yet been stopped in practice.
This is the main reason why absolute zero temperatures cannot be reached. There are still debates about the consequences of this process. From the point of view of thermodynamics, this limit is unattainable, since the thermal movement of atoms and molecules stops completely, and a crystal lattice is formed.
Representatives of quantum physics envision the presence of minimum zero oscillations at absolute zero temperatures.
What is the value of absolute zero temperature and why it cannot be achieved
At the General Conference on Weights and Measures, a reference or reference point was established for the first time for measuring instruments that determine temperature indicators.
Currently, in the International System of Units, the reference point for the Celsius scale is 0°C for freezing and 100°C for boiling, the value of absolute zero temperatures is equal to −273.15°C.
Using temperature values on the Kelvin scale according to the same International System of Units, boiling of water will occur at the reference value of 99.975 ° C, absolute zero is equal to 0. On the Fahrenheit scale the indicator corresponds to -459.67 degrees.
But, if these data are obtained, why then is it impossible to achieve absolute zero temperatures in practice? For comparison, we can take the well-known speed of light, which is equal to the constant physical value of 1,079,252,848.8 km/h.
However, this value cannot be achieved in practice. It depends on the transmission wavelength, the conditions, and the required absorption of a large amount of energy by the particles. To obtain the value of absolute zero temperatures, a large output of energy is required and the absence of its sources to prevent it from entering atoms and molecules.
But even in conditions of complete vacuum, scientists were unable to obtain either the speed of light or absolute zero temperatures.
Why is it possible to reach approximately zero temperatures, but not absolute zero?
What will happen when science can come close to achieving the extremely low temperature of absolute zero remains only in the theory of thermodynamics and quantum physics. What is the reason why absolute zero temperatures cannot be achieved in practice.
All known attempts to cool a substance to the lowest limit due to maximum energy loss led to the fact that the heat capacity of the substance also reached a minimum value. The molecules were simply no longer able to give up the remaining energy. As a result, the cooling process stopped without reaching absolute zero.
When studying the behavior of metals under conditions close to absolute zero temperatures, scientists found that a maximum decrease in temperature should provoke a loss of resistance.
But the cessation of the movement of atoms and molecules only led to the formation of a crystal lattice, through which passing electrons transferred part of their energy to stationary atoms. Again, it was not possible to reach absolute zero.
In 2003, the temperature was only half a billionth of 1°C short of absolute zero. NASA researchers used a Na molecule to conduct experiments, which was always in a magnetic field and gave up its energy.
The closest achievement was achieved by scientists at Yale University, who in 2014 achieved a figure of 0.0025 Kelvin. The resulting compound, strontium monofluoride (SrF), lasted only 2.5 seconds. And in the end it still disintegrated into atoms.
The term “temperature” appeared at a time when physicists thought that warm bodies consisted of more of a specific substance - caloric - than the same bodies, but cold ones. And temperature was interpreted as a value corresponding to the amount of caloric in the body. Since then, the temperature of any body has been measured in degrees. But in fact it is a measure of the kinetic energy of moving molecules, and, based on this, it should be measured in Joules, in accordance with the System of Units C.
The concept of “absolute zero temperature” comes from the second law of thermodynamics. According to it, the process of heat transfer from a cold body to a hot one is impossible. This concept was introduced by the English physicist W. Thomson. For his achievements in physics, he was given the title of nobility “Lord” and the title “Baron Kelvin”. In 1848, W. Thomson (Kelvin) proposed using a temperature scale in which he took absolute zero temperature, corresponding to extreme cold, as the starting point, and took degrees Celsius as the division value. The Kelvin unit is 1/27316 of the temperature of the triple point of water (about 0 degrees C), i.e. temperature at which pure water immediately exists in three forms: ice, liquid water and steam. temperature is the lowest possible low temperature at which the movement of molecules stops and it is no longer possible to extract thermal energy from a substance. Since then, the absolute temperature scale has been named after him.
Temperature is measured on different scales

The most commonly used temperature scale is called the Celsius scale. It is built on two points: the temperature of water from liquid to steam and water to ice. A. Celsius in 1742 proposed dividing the distance between reference points into 100 intervals, and taking water as zero, with the freezing point as 100 degrees. But the Swede K. Linnaeus suggested doing the opposite. Since then, water has frozen at zero degrees A. Celsius. Although it should boil exactly at Celsius. Absolute zero Celsius corresponds to minus 273.16 degrees Celsius.
There are several more temperature scales: Fahrenheit, Reaumur, Rankin, Newton, Roemer. They have different division prices. For example, the Reaumur scale is also built on the reference points of boiling and freezing of water, but it has 80 divisions. The Fahrenheit scale, which appeared in 1724, is used in everyday life only in some countries of the world, including the USA; one is the temperature of the mixture of water ice and ammonia and the other is the temperature of the human body. The scale is divided into one hundred divisions. Zero Celsius corresponds to 32 Conversion of degrees to Fahrenheit can be done using the formula: F = 1.8 C + 32. Reverse conversion: C = (F - 32)/1.8, where: F - degrees Fahrenheit, C - degrees Celsius. If you are too lazy to count, go to an online service for converting Celsius to Fahrenheit. In the box, enter the number of degrees Celsius, click "Calculate", select "Fahrenheit" and click "Start". The result will appear immediately.

Named after the English (more precisely Scottish) physicist William J. Rankin, who was a contemporary of Kelvin and one of the creators of technical thermodynamics. There are three important points in his scale: the beginning is absolute zero, the freezing point of water is 491.67 degrees Rankine and the boiling point of water is 671.67 degrees. The number of divisions between the freezing of water and its boiling for both Rankine and Fahrenheit is 180.
Most of these scales are used exclusively by physicists. And 40% of American high school students surveyed today said that they do not know what absolute zero temperature is.

 Absolute zero(absolute zero) – the beginning of the absolute temperature, starting from 273.16 K below the triple point of water (the equilibrium point of three phases - ice, water and water vapor); At absolute zero, the movement of molecules stops, and they are in a state of “zero” motion. Or: the lowest temperature at which a substance contains no thermal energy.
Absolute zero(absolute zero) – the beginning of the absolute temperature, starting from 273.16 K below the triple point of water (the equilibrium point of three phases - ice, water and water vapor); At absolute zero, the movement of molecules stops, and they are in a state of “zero” motion. Or: the lowest temperature at which a substance contains no thermal energy.
Absolute zero Start absolute temperature reading. Corresponds to -273.16 ° C. At present, in physical laboratories it has been possible to obtain a temperature exceeding absolute zero by only a few millionths of a degree, but according to the laws of thermodynamics, it is impossible to achieve it. At absolute zero, the system would be in a state with the lowest possible energy (in this state, atoms and molecules would perform “zero” vibrations) and would have zero entropy (zero disorder). The volume of an ideal gas at the point of absolute zero must be equal to zero, and to determine this point, the volume of real helium gas is measured at sequential lowering the temperature until it liquefies at low pressure (-268.9 ° C) and extrapolates to the temperature at which the volume of gas would become zero in the absence of liquefaction. Absolute temperature thermodynamic scale is measured in kelvins, denoted by the symbol K. Absolute thermodynamic the scale and the Celsius scale are simply offset from one another and are related by the ratio K = °C + 273.16 °.
Story
The word “temperature” arose in those days when people believed that more heated bodies contained a larger amount of a special substance - caloric - than less heated ones. Therefore, temperature was perceived as the strength of a mixture of body matter and caloric. For this reason, the units of measurement for the strength of alcoholic beverages and temperature are called the same - degrees.
Since temperature is the kinetic energy of molecules, it is clear that it is most natural to measure it in energy units (i.e. in the SI system in joules). However, temperature measurement began long before the creation of the molecular kinetic theory, so practical scales measure temperature in conventional units - degrees.
Kelvin scale
Thermodynamics uses the Kelvin scale, in which temperature is measured from absolute zero (the state corresponding to the minimum theoretically possible internal energy of a body), and one kelvin is equal to 1/273.16 of the distance from absolute zero to the triple point of water (the state in which ice, water and water pairs are in equilibrium). Boltzmann's constant is used to convert kelvins into energy units. Derived units are also used: kilokelvin, megakelvin, millikelvin, etc.
Celsius
In everyday life, the Celsius scale is used, in which 0 is the freezing point of water, and 100° is the boiling point of water at atmospheric pressure. Since the freezing and boiling points of water are not well defined, the Celsius scale is currently defined using the Kelvin scale: a degree Celsius is equal to a kelvin, absolute zero is taken to be −273.15 °C. The Celsius scale is practically very convenient because water is very common on our planet and our life is based on it. Zero Celsius is a special point for meteorology, since the freezing of atmospheric water changes everything significantly.
Fahrenheit
In England and especially in the USA, the Fahrenheit scale is used. This scale divides the interval from the temperature of the coldest winter in the city where Fahrenheit lived to the temperature of the human body into 100 degrees. Zero degrees Celsius is 32 degrees Fahrenheit, and a degree Fahrenheit is equal to 5/9 degrees Celsius.
The current definition of the Fahrenheit scale is as follows: it is a temperature scale in which 1 degree (1 °F) is equal to 1/180th the difference between the boiling point of water and the melting temperature of ice at atmospheric pressure, and the melting point of ice is +32 °F. Temperature on the Fahrenheit scale is related to temperature on the Celsius scale (t °C) by the ratio t °C = 5/9 (t °F - 32), 1 °F = 5/9 °C. Proposed by G. Fahrenheit in 1724.
Reaumur scale
Proposed in 1730 by R. A. Reaumur, who described the alcohol thermometer he invented.
The unit is the degree Reaumur (°R), 1 °R is equal to 1/80 of the temperature interval between the reference points - the melting temperature of ice (0 °R) and the boiling point of water (80 °R)
1 °R = 1.25 °C.
Currently, the scale has fallen out of use; it survived longest in France, the author’s homeland.
Comparison of temperature scales
| Description | Kelvin | Celsius | Fahrenheit | Newton | Reaumur |
| Absolute zero | −273.15 | −459.67 | −90.14 | −218.52 | |
| Melting temperature of a mixture of Fahrenheit (salt and ice in equal quantities) | 0 | −5.87 | |||
| Freezing point of water (normal conditions) | 0 | 32 | 0 | ||
| Average human body temperature¹ | 36.8 | 98.2 | 12.21 | ||
| Boiling point of water (normal conditions) | 100 | 212 | 33 | ||
| Solar surface temperature | 5800 | 5526 | 9980 | 1823 |
Normal human body temperature is 36.6 °C ±0.7 °C, or 98.2 °F ±1.3 °F. The commonly quoted value of 98.6°F is an exact conversion to Fahrenheit of the 19th century German value of 37°C. Since this value is not within the range of normal temperature according to modern concepts, we can say that it contains excessive (incorrect) accuracy. Some values in this table have been rounded.
Comparison of Fahrenheit and Celsius scales
(o F- Fahrenheit scale, oC- Celsius scale)
| oF | oC | oF | oC | oF | oC | oF | oC | |||
| -459.67 -450 -400 -350 -300 -250 -200 -190 -180 -170 -160 -150 -140 -130 -120 -110 -100 -95 -90 -85 -80 -75 -70 -65 | -273.15 -267.8 -240.0 -212.2 -184.4 -156.7 -128.9 -123.3 -117.8 -112.2 -106.7 -101.1 -95.6 -90.0 -84.4 -78.9 -73.3 -70.6 -67.8 -65.0 -62.2 -59.4 -56.7 -53.9 | -60 -55 -50 -45 -40 -35 -30 -25 -20 -19 -18 -17 -16 -15 -14 -13 -12 -11 -10 -9 -8 -7 -6 -5 | -51.1 -48.3 -45.6 -42.8 -40.0 -37.2 -34.4 -31.7 -28.9 -28.3 -27.8 -27.2 -26.7 -26.1 -25.6 -25.0 -24.4 -23.9 -23.3 -22.8 -22.2 -21.7 -21.1 -20.6 | -4 -3 -2 -1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 | -20.0 -19.4 -18.9 -18.3 -17.8 -17.2 -16.7 -16.1 -15.6 -15.0 -14.4 -13.9 -13.3 -12.8 -12.2 -11.7 -11.1 -10.6 -10.0 -9.4 -8.9 -8.3 -7.8 -7.2 | 20 21 22 23 24 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 125 150 200 | -6.7 -6.1 -5.6 -5.0 -4.4 -3.9 -1.1 1.7 4.4 7.2 10.0 12.8 15.6 18.3 21.1 23.9 26.7 29.4 32.2 35.0 37.8 51.7 65.6 93.3 |
To convert degrees Celsius to Kelvin, you must use the formula T=t+T 0 where T is the temperature in kelvins, t is the temperature in degrees Celsius, T 0 =273.15 kelvins. The size of a degree Celsius is equal to a kelvin.