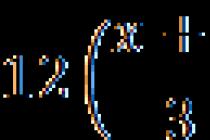Modern chemistry is a science that operates in a large number of reagents. It may be salts, reagents, alkali. But the most numerous group is acids. These are complex compounds based on hydrogen. At the same time, third-party atoms here can be replaced by metal atoms. Acids are used in various branches of human life. For example, in medicine, food industry, in the production of household goods. That is why it should be especially carefully studying this group of reagents.
Basic information about nitric acid
This is a strong reagent, which refers to the discharge of single-maintain acids. It looks like an ordinary transparent liquid. Sometimes there is a yellowish tint. This is due to the fact that with a warm temperature on the surface, nitrogen oxide accumulates. Nitrogen dioxide can also manifest itself in the form of a brown sediment. But it happens under the sunny rays. With any contact with air, the acid begins to smoke. In addition, normally enters the reaction with metals. It is perfectly soluble in water, but in the case of ether there are a number of restrictions.
What form of release exist? Total separated by two - ordinary (concentration 65-68%) and smoky (at least 85%). At the same time, the color of smoke can vary greatly. If the concentration is 86-95%, then it is white. Percentage above? Then you will see a red.
Process of receipt
Today it does not differ both in the case of a strong and weak concentration. It can be divided into several stages.
Crystalline oxidation of ammonia synthetics occurs.
It is necessary to wait when nitrous gases are formed.
All water that was in the composition is absorbed.
At the final stage, it is necessary to wait until the acid reaches the necessary concentration.
How is storage and transportation?
This reagent does not relate to the category of particularly aggressive. Therefore, the requirements for storage and transportation are not so much. Keep the acid is required in sealed tanks made of aluminum or chrome steel. Laboratory glass is also suitable. As for the tanks, they must be a mark "DANGER". The same applies to the small Tar.
Precautions when using
This chemical reagent refers to strong acids. It has the III class of danger. Those persons who are allowed to work with this substance must pass the appropriate instruction. Indoors must be in special clothing. It includes jumpsuit, mittens, respirators, glasses. Individual means of protecting respiratory and vision organs are needed. The consequences in non-compliance with safety requirements may be serious. If the acid falls on the skin, it will lead to the formation of burns and ulcers. Inspiration her? Then you will choose or even get the swelling of the lungs. So in laboratories, it is necessary to organize constant control, ask for employees to go on safety instructions.
Where is nitric acid applied?
Due to its chemical properties, this acid is used in many industries. Separately select several. First of all it is the industry. With the help of it, it is possible to easily synthesize artificial fiber. In addition, often nitric acid is the main component in the manufacture of engine oil. Surely you know that it is used in metallurgy. With the help of it you can dissolve and stretch metals. There is a special industrial nitrogen acid, which is better coping with the solution of the tasks described.
Application in everyday life
It makes money from it that make it possible to effectively clean jewelry at home. But you need to be extremely careful, prevent your skin contacts with skin. With drip watering, nitric acid can be applied as a cleaner. Concentrations of 60% will be enough to get rid of salts or dissolve the precipitate in the drip system.
What is the use of medicine?
If you look at the composition of some medical products, you will see that nitric acid is contained. For example, 30% is used to combat warts. Also, this component is also added to the means of combating ulceated diseases. This is an excellent antiseptic with knitting properties.
Usage in agriculture
The agronomas need mineral fertilizers in order to make a harvest richer. As part of some of them, nitric acid can be found. But it is necessary to clearly count the dose so that the obtained vegetables and fruits make any harm to health. If the acids are too much, nitrates will accumulate in cultures. Several types of acid-based fertilizers can be distinguished: amide, ammonia, nitrate.
But this reagent has salts that are used even more often in agriculture. They are added to some drugs that give animals.
What can be said in conclusion?
As you can see, nitric acid is a very important component used in a huge amount of industries. Without it, it would be impossible to present modern life. And chemists on a regular basis are invented where you can still use this reagent.
In contact with
Nitric acid - important but dangerous chemical reagent
Chemical reagents, laboratory equipment and appliances , and laboratory glassware Or from other materials are components of any modern industrial or research laboratory. In this list, like many centuries ago, a special place is occupied by substances and compounds, as they are the main chemical base, without which anyone, even the simplest experiment or analysis, is impossible.
Modern chemistry has a huge amount of chemical reagents: alkalis, acids, reagents, salts and others. Among them are acids - the most common group. Acids are complex hydrogen-containing compounds whose atoms can be replaced by metal atoms. The scope of their use is extensive. It covers many industries: chemical, engineering, oil refineries, food, as well as medicine, pharmacology, cosmetology; Widely used in everyday life.
Nitric acid and its definition
 Refers to mono-zero acids and is a strong reagent. It is a transparent fluid that may have a yellowish tint with a long storage of it in a warm room, since with a plus (room) temperature, nitrogen oxides accumulate. When heated or interacting with straight solar rays, brown color is purchased due to the process of isolated nitrogen dioxide. When contacting with air smokes. This acid is a strong oxidizing agent with a sharp unpleasant odor, which reacts with most metals (with the exception of platinum, rhodium, gold, tantalum, iridium and some others), turning them into oxides or nitrates. This acid is well soluble in water, and in any relations, limited - on the air.
Refers to mono-zero acids and is a strong reagent. It is a transparent fluid that may have a yellowish tint with a long storage of it in a warm room, since with a plus (room) temperature, nitrogen oxides accumulate. When heated or interacting with straight solar rays, brown color is purchased due to the process of isolated nitrogen dioxide. When contacting with air smokes. This acid is a strong oxidizing agent with a sharp unpleasant odor, which reacts with most metals (with the exception of platinum, rhodium, gold, tantalum, iridium and some others), turning them into oxides or nitrates. This acid is well soluble in water, and in any relations, limited - on the air.
The form of the production of nitric acid depends on its concentration:
- ordinary - 65%, 68%;
- Smoky - 86% or more. The color of "smoke" can be white if the concentration ranges from 86% to 95%, or red - over 95%.
Obtaining
Currently, the production of strongly or weakly concentrated nitric acid passes the following steps:
1. The process of catalytic oxidation of synthetic ammonia;
2. As a result - obtaining a mixture of nitrous gases;
3. water absorption;
4. The process of concentration of nitric acid.
Storage and transportation
This reagent is the most aggressive acid,  therefore, the following requirements are put forward for its transportation and storage:
therefore, the following requirements are put forward for its transportation and storage:
- store and transport in special hermetically closed tanks from chromium steel or aluminum, as well as in bottles of laboratory glass.
Each container is marked with the inscription "DANGER".
Where does the chemical reagent apply?
The scope of nitric acid is currently huge. It covers many industries, such as:
- chemical (manufacture of explosives, organic dyes, plastics, sodium, potassium, plastics, some types of acids, artificial fiber);
- agricultural (production of nitrogen mineral fertilizers or nitrates);
- metallurgical (dissolution and etching of metals);
- pharmacological (included in the preparations for removing skin formations);
- jewelry production (determination of the purity of precious metals and alloys);
- military (included in the composition of explosives as a nitrative reagent);
- rocket and cosmic (one of the components of rocket fuel);
- medicine (for causing warts and other skin formations).
Precautions
When working with nitric acid, it is necessary to take into account that this chemical reagent is a strong acid that refers to substances of 3 hazard class. For employees of laboratories, as well as persons admitted to work with similar substances, there are special rules. In order to avoid direct contact with the reagent, all the work is strictly in special clothing, which includes: acid-protective mittens and shoes, jumpsuit, nitrile gloves, as well as glasses and respirators, as the means of protecting the respiratory and vision organs. Failure to comply with these requirements can lead to the most serious consequences: when burning burns, ulcers, and when inhaling, poisoning, right up to edema of the lungs.
: Monohydrate (HNO 3 · H 2 O) and trihydrate (HNO 3 · 3H 2 O).
Physical and physico-chemical properties
Phase diagram of aqueous nitric acid solution.
Nitrogen in nitric acid fourhound, the degree of oxidation is +5. Nitric acid is a colorless, fluid smoking in air, melting point -41,59 ° C, boiling +82.6 ° C with partial decomposition. The solubility of nitric acid in water is not limited. HNO 3 aqueous solutions with a mass fraction of 0.95-0.98 are called "smoking nitric acid", with a mass fraction of 0.6-0.7 - concentrated nitric acid. With water forms azeotropic mixture (mass fraction of 68.4%, d. 20 \u003d 1.41 g / cm, T kip \u003d 120.7 ° C)
When crystallization from aqueous solutions, nitric acid forms crystallohydrates:
- hNO 3 · H 2 O, T MONO 3 · H 2 O, T PL \u003d -37,62 ° C
- hNO 3 · 3H 2 O, T PL \u003d -18,47 ° C
Till nitric acid forms two crystal modifications:
- monoclinic, spatial group P. 2 1 / a, a. \u003d 1,623 nm, b. \u003d 0.857 nm, c. \u003d 0.631, β \u003d 90 °, z \u003d 16;
Monohydrate forms Rhombic Singonia crystals, spatial group P. Na2 a. \u003d 0.631 nm, b. \u003d 0.869 nm, c. \u003d 0.544, z \u003d 4;
The density of aqueous solutions of nitric acid as a function of its concentration is described by the equation
where D is the density in g / cm ³, C is the mass fraction of acid. This formula poorly describes the density behavior at a concentration of more than 97%.
Chemical properties
HNO 3 highly concentrated is usually a brown color due to the decomposition process that happens:
When heating nitric acid decomposes by the same reaction. Nitric acid can be distilled (without decomposition) only under reduced pressure (the specified boiling point at atmospheric pressure was found extrapolation).
c) displaces weak acids from their salts:
When boiling or under the action of light, nitric acid is partially decomposed:
Nitric acid at any concentration exhibits the properties of the oxidant acid, while nitrogen is restored to the degree of oxidation from +4 to -3. The depth of recovery depends primarily on the nature of the reducing agent and on the concentration of nitric acid. As an acid oxidizer, HNO 3 interacts:
Nitrate
Nitric acid is a strong acid. Its salts are nitrates - are obtained by the action of HNO 3 on metals, oxides, hydroxides or carbonates. All nitrates are well soluble in water. Nitrate ion in water is not hydrolyzed.
Salts of nitric acid during heating irreversibly decompose, and the composition of the decomposition products is determined by the cation:
a) Nitrates of metals standing in a row of voltages to the left of magnesium:
b) nitrates of metals located in a row of stresses between magnesium and copper:
c) nitrates of metals located in a row of stresses to the right:
Nitrates in aqueous solutions practically do not exhibit oxidative properties, but at high temperatures in solid state are strong oxidizers, for example, when fusing solids:
Historical information
The method of obtaining dilute nitric acid by dry distillery of nashelters with alum and copper vitrios was apparently described by the Treatises of Jing (Gebra in Latinized Translations) in the VIII century. This method with those or other modifications, the most significant of which was the replacement of the copper municipal of iron, was used in the European and Arabic alchemy until the XVII century.
In the XVII century, Glauber proposed a method for obtaining volatile acids by the reaction of their salts with concentrated sulfuric acid, including nitric acid from potash nitality, which made it possible to introduce concentrated nitric acid into chemical practice and learn its properties. Method
Nitric acid is one of the strongest acids, similar to transparent liquid, with a slightly yellowish tinge, toxic substance. In the interaction of acid vapor with an atmosphere, nitric acid slightly "smokes", by transforming small foggy drops. Among other things, the acid is a wonderful oxidizing agent, extensively applied in different fields of industry. Nitric acid is used for the production of nitrate, mineral fertilizers and so on. Even in the manifestation of photographs, it is added to acidify, in the metallurgical industry - for dissolving metals, and in printing, need an acid for etching printing. However, for the human body, it is explicit harm, and working with it, the means of individual protection must necessarily need.
Spheres of nitric acid
The essence of nitric acid in the national economy is simply undeniable, it is difficult to imagine, probably an agricultural firm that, engaged in the cultivation of cultures, would not use this type of acids. Nitric acid is also freely used in the military industry, oxidizing the fuel for rockets, dilutes various substances. In the manufacture of drugs and dyes, it is also not to do without nitric acid, but in jewelry, it simply does not have equal, in terms of determining the part gold In general gold alloy.
The total share of the need for agricultural enterprises in nitric acid is estimated about 80%, from the overall level of the needs of the national economy, and only 10% fall into the military industry. Despite the interchangeability of acids in production, as well as the degree of moral wear of the equipment for its production, the need is growing every year. The greater advantage of this type of acid is the ability to mix with water in any concentrations, dissociating thus on ions. Indicators of industrial growth and needs from year to year are growing in geometric progression.