In turn, sedimentary deposits, in accordance with the conditions of their formation, are divided into synthetic and epigenetic. In this case, syngenetic deposits were formed simultaneously with rocks containing gypsum and anhydrite, as a result of chemical precipitation reactions from solutions. And epigenetic deposits of gypsum arose as a result of hydration of previously formed anhydrite under the influence of groundwater.
Deposits of gypsum and anhydrite in syngenetic sedimentary deposits have the form of lenses and layers with a thickness of up to 20 m or more. Gypsum deposits in epigenetic deposits are layers and lenses complicated by the development of internal tectonics (intrastratal folding, bulges, constrictions) and near-contact zones of crushing and brecciation, since the process of anhydrite hydration is inevitably accompanied by an increase in rock volume by approximately 30%.
Almost all large deposits of gypsum mineral raw materials in Russia are sedimentary deposits. Residual deposits result from the accumulation of gypsum and anhydrite as residual products from the leaching of readily soluble minerals in salt deposits, so-called "gypsum hat" deposits. Metasomatic deposits are formed due to the replacement of carbonate rocks with gypsum when CaCo3 is exposed to sulfuric acid waters. There are practically no industrial deposits of this type in Russia. Weathering deposits are formed due to the dissolution of gypsum dispersed in sedimentary rocks, the transfer of its solutions by ground and surface waters, followed by deposition mixed with sand, clay and calcareous particles in the form of so-called clay gypsum, earthy gypsum, drywall, etc.
Deposits of gypsum and anhydrite are found in most geological systems - from the Cambrian period to the Quaternary. In Russia, industrially significant gypsum deposits are found in sediment layers that formed during the Cambrian, Devonian, Carboniferous, Permian, Jurassic and Quaternary geological epochs of the Earth’s existence. Moreover, over 55% of the reserves of gypsum and anhydrite were formed in the Carboniferous period, about 32% in the Permian period and 10% in the Devonian period. 3% of gypsum-bearing rocks were formed in the Jurassic and Cambrian periods and only 0.3% in the Quaternary.
State balance of mineral reserves of Russia as of January 1, 2003, million tons, which are distributed throughout the territory of our country. According to table. 1. Of these, only 24 fields are considered as being developed, which is 28%
table 1.
Federal District | Number of deposits | Reserves | ||
| Total | Exploited | Million tons | Share of total reserves in Russia, % | |
| Central | 6 | 1 | 1850,7 | 56,5 |
| Northwestern | 3 | - | 47,1 | 1,4 |
| Southern | 20 | 6 | 308,6 | 9,4 |
| Privolzhsky | 38 | 12 | 851,8 | 26,0 |
| Ural | 4 | 1 | 35,3 | 1,1 |
| Siberian | 11 | 3 | 163,4 | 5,0 |
| Far Eastern | 4 | 1 | 19,0 | 0,6 |
| Total in Russia | 86 | 24 | 3275,9 | 100,0 |
As can be seen from the data given in table. 1 The Central Federal District has the largest reserves, since the existing 6 deposits are very large, and more than half of Russia’s gypsum raw materials are concentrated in them. The Volga and Southern Federal Districts have significant reserves (about 35% in total). Most of the existing 86 deposits of gypsum raw materials have relatively small reserves. Only 19 can be classified as large deposits, that is, with reserves of more than 25 million tons, but they account for about 90% of all gypsum in Russia.
The majority of all reserves (75%) are concentrated in the 9 largest deposits with reserves of more than 100 million tons each - Novomoskovskoye, Pavlovskoye, Skuratovskoye, Bolokhovskoye, Pletnevskoye, Baskunchakskoye, Lazinskoye, Poretskoye and Obolenskoye.
The payable strata of most (79) deposits is a mixture of gypsum and anhydrite, usually with a significant predominance of gypsum (up to 90%). Anhydrite accounts for less than 10% of the total reserves. The world's proven reserves of gypsum amount to more than 7,500 million tons, of which approximately half are in Russia. World production of natural raw materials is approximately 110 million tons. Currently, the volume of gypsum production in Russia has reached approximately 6 million tons per year, which is 5-6% of world production. Three quarters of the world's gypsum production comes from 9 countries: the USA, Thailand, Canada, Iran, China, Spain, Mexico, Japan and France. At the same time, in the USA. Among the CIS countries, the largest volume of production of gypsum raw materials takes place in Ukraine and Tajikistan. Now it is approximately 100 thousand tons/year in each of them. The reserves of most of the fields being developed in Russia make it possible to significantly increase the volume of raw material production. Of the 62 undeveloped fields, only 9 have significant reserves, but 6 of them have reserves of over 100 million tons each (see Table 2).
Original taken from zizis
to Gips, BelAZ and Marble Lake.
Let me tell you how gypsum is mined. If you think that gypsum mining does not concern you, then you are very mistaken. Gypsum is almost everywhere: in building materials, hospitals, jewelry, works of art, and even, as they say, in McDonald's buns. Well, I won’t say anything about buns, I don’t use them, but the fact that this unique hypoallergenic substance haunts us throughout our lives and a little later is a fact.
But I will show where it comes from using the example of the KNAUF Gips Baskunchak company.
02.
Well, let's talk briefly about the process itself, and then photos of large and powerful machines.
This is the quarry where gypsum is mined. Development for the needs of the Volga region began in 1897, and the quarry itself was formed in 1927. A gypsum plant was built in 1932, and since 1949, excavator mining was introduced at the quarry.
At the enterprise, old Soviet mastodons and new foreign cars work side by side. As we were assured by the company's management, Soviet technology will soon be completely replaced by modern foreign ones. As for me, it’s an excellent technique, electric. Do you see a pebble in the bucket? It is approximately three meters in diameter.
The broken pieces of rock are loaded into large 20-ton dump trucks. BelAZ trucks are found here
Yes, that's it. After the collapse of the USSR, the plant became JSC Mineral, after which in 1998 the shares were purchased by the German company Knauf.
And here are the BelAZs. Dump trucks deliver rock to the stone crusher. The speed in the quarry is no more than 60 km/h, as in the city.
Large pieces of rock are loaded into a rock crusher. One car is unloading, the second is turning around for unloading, the third is already approaching.
Receiving hole of the stone crusher. It's scary to imagine what's going on inside.
Huge pieces are crushed into 5-10 mm pieces and placed on a conveyor. Unfortunately, I don’t know the name of this building.
And they are poured into piles for further loading and transportation. The piles, it must be said, are not small, the height of a three-story building. I tried to climb onto one of them, but was stopped in the middle, it was impossible.
However, I still have this photograph as a keepsake.
This is what the stone crusher looks like from the outside.
Unfortunately, internal security rules did not allow the general director of the enterprise to show us the entire production cycle, but he explained it to us in great detail and popularly in words.
These are additives for the production of various mixtures. They are introduced into plaster according to certain secret recipes, it all depends on the requirements for the material.
15.
After grinding the gypsum and mixing it with additives, a ready-made construction or facing mixture is obtained. It is packaged, stamped and the products are packed onto pallets.
16.
Local residents work in production. And it’s very difficult to hold on to a place here; a stable year-round salary and any kind of social package are very difficult to find in these places.
17.
Dmitry Andreevich spoke about several degrees of product protection against counterfeiting. Most of these defenses will only be seen by a professional, but we may need a couple of obvious ones. A sticker is now made on the front side of the bag, changing its color when touched by hand.
18.
If you buy products from Knauf, or almost any major manufacturer, look at the stamp. The date and time of production must be indicated there, down to the second. If all the stamps in one batch are the same, this is an obvious fake.
19.
Panorama, click on larger version.
Let's go back to the quarry and look at the nature of this place and the big machines.
20.
“Wild lifeless desert”... This seems to be the only green bush I saw there.
21.
Behind these stones lies the village of Sredny Baskunchak.
22.
Inspection of the property.
23.
And now the cars. There is nothing to tell about them, the yellow ones are large and powerful. Therefore, further without text.
27.
Here's another handsome guy. Electrically powered.
28.
The roads in the quarry are constantly flooded with water from Marmara Lake. The water there is clean, you can see it even in the photo.
29.
This is the lake. Unfortunately, free access to it is closed - production...
30.
Our guide Olga Sergeevna examines the cast. I didn't expect him to be so cool. Soft warm stone, almost plush
31.
This is what the quarry looks like from the outside. You can't see anything behind the waste heaps.
32.
We take a farewell look at Nizhny Baskunchak and the lake, say “goodbye” to them and move on.
Gypsum- mineral, hydrous calcium sulfate. The fibrous variety of gypsum is called selenite, and the granular variety is called alabaster. One of the most common minerals; the term is also used to designate the rocks it consists of. Gypsum is also commonly called a building material obtained by partial dehydration and grinding of the mineral. The name comes from the Greek. gypsos, which in ancient times meant both plaster itself and chalk. A dense snow-white, cream or pink fine-grained variety of gypsum known as alabaster
See also:
STRUCTURE
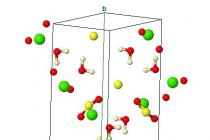
 Chemical composition - Ca × 2H 2 O. Monoclinic system. The crystal structure is layered; two sheets of anionic 2- groups, closely associated with Ca 2+ ions, form double layers oriented along the (010) plane. H 2 O molecules occupy spaces between these double layers. This easily explains the very perfect cleavage characteristic of gypsum. Each calcium ion is surrounded by six oxygen ions belonging to SO 4 groups and two water molecules. Each water molecule binds a Ca ion to one oxygen ion in the same bilayer and to another oxygen ion in the adjacent layer.
Chemical composition - Ca × 2H 2 O. Monoclinic system. The crystal structure is layered; two sheets of anionic 2- groups, closely associated with Ca 2+ ions, form double layers oriented along the (010) plane. H 2 O molecules occupy spaces between these double layers. This easily explains the very perfect cleavage characteristic of gypsum. Each calcium ion is surrounded by six oxygen ions belonging to SO 4 groups and two water molecules. Each water molecule binds a Ca ion to one oxygen ion in the same bilayer and to another oxygen ion in the adjacent layer. PROPERTIES
 The color varies, but usually white, gray, yellow, pink, etc. Pure transparent crystals are colorless. Impurities can be painted in different colors. The color of the dash is white. The luster of the crystals is glassy, sometimes with a pearlescent tint due to microcracks of perfect cleavage; in selenite it is silky. Hardness 2 (Mohs scale standard). The cleavage is very perfect in one direction. Thin crystals and fusion plates are flexible. Density 2.31 - 2.33 g/cm3.
The color varies, but usually white, gray, yellow, pink, etc. Pure transparent crystals are colorless. Impurities can be painted in different colors. The color of the dash is white. The luster of the crystals is glassy, sometimes with a pearlescent tint due to microcracks of perfect cleavage; in selenite it is silky. Hardness 2 (Mohs scale standard). The cleavage is very perfect in one direction. Thin crystals and fusion plates are flexible. Density 2.31 - 2.33 g/cm3.
It has noticeable solubility in water. A remarkable feature of gypsum is the fact that its solubility with increasing temperature reaches a maximum at 37-38°, and then drops quite quickly. The greatest decrease in solubility occurs at temperatures above 107° due to the formation of “hemihydrate” - CaSO 4 × 1/2H 2 O.
At 107°C, it partially loses water, turning into white alabaster powder (2CaSO 4 × H 2 O), which is noticeably soluble in water. Due to the smaller number of hydration molecules, alabaster does not shrink during polymerization (increases in volume by approximately 1%). Under item tr. loses water, splits and fuses into white enamel. On coal in a reducing flame it produces CaS. It dissolves much better in water acidified with H 2 SO 4 than in pure water. However, at a concentration of H 2 SO 4 above 75 g/l. solubility drops sharply. Very slightly soluble in HCl.
MORPHOLOGY
 Crystals, due to the predominant development of faces (010), have a tabular, rarely columnar or prismatic appearance. Of the prisms, the most common are (110) and (111), sometimes (120), etc. The faces (110) and (010) often have vertical hatching. Fusion twins are common and come in two types: 1) Gallic by (100) and 2) Parisian by (101). It is not always easy to distinguish them from each other. Both of them resemble a dovetail. Gallic twins are characterized by the fact that the edges of the prism m (110) are located parallel to the twin plane, and the edges of the prism l (111) form a reentrant angle, while in Parisian twins the edges of the prism Ι (111) are parallel to the twin seam.
Crystals, due to the predominant development of faces (010), have a tabular, rarely columnar or prismatic appearance. Of the prisms, the most common are (110) and (111), sometimes (120), etc. The faces (110) and (010) often have vertical hatching. Fusion twins are common and come in two types: 1) Gallic by (100) and 2) Parisian by (101). It is not always easy to distinguish them from each other. Both of them resemble a dovetail. Gallic twins are characterized by the fact that the edges of the prism m (110) are located parallel to the twin plane, and the edges of the prism l (111) form a reentrant angle, while in Parisian twins the edges of the prism Ι (111) are parallel to the twin seam. It occurs in the form of colorless or white crystals and their intergrowths, sometimes colored by inclusions and impurities captured by them during growth in brown, blue, yellow or red tones. Characteristic are intergrowths in the form of a “rose” and twins - the so-called. "swallowtails"). It forms veinlets of a parallel-fibrous structure (selenite) in clayey sedimentary rocks, as well as dense, continuous fine-grained aggregates resembling marble (alabaster). Sometimes in the form of earthy aggregates and cryptocrystalline masses. Also makes up the cement of sandstones.
Pseudomorphoses of calcite, aragonite, malachite, quartz, etc. on gypsum are common, as are pseudomorphs of gypsum on other minerals.
ORIGIN
 A widespread mineral, it is formed in natural conditions in various ways. The origin is sedimentary (typical marine chemogenic sediment), low-temperature hydrothermal, found in karst caves and solfataras. Precipitates from sulfate-rich aqueous solutions during the drying out of sea lagoons and salt lakes. Forms layers, layers and lenses among sedimentary rocks, often in association with anhydrite, halite, celestine, native sulfur, sometimes with bitumen and oil. It is deposited in significant quantities by sedimentation in lake and sea salt-bearing dying basins. In this case, gypsum, along with NaCl, can be released only in the initial stages of evaporation, when the concentration of other dissolved salts is not yet high. When a certain value of salt concentration is reached, in particular NaCl and especially MgCl 2, anhydrite will crystallize instead of gypsum and then other, more soluble salts, i.e. The gypsum in these basins must belong to earlier chemical sediments. Indeed, in many salt deposits, layers of gypsum (as well as anhydrite), interbedded with layers of rock salt, are located in the lower parts of the deposits and in some cases are underlain only by chemically precipitated limestones.
A widespread mineral, it is formed in natural conditions in various ways. The origin is sedimentary (typical marine chemogenic sediment), low-temperature hydrothermal, found in karst caves and solfataras. Precipitates from sulfate-rich aqueous solutions during the drying out of sea lagoons and salt lakes. Forms layers, layers and lenses among sedimentary rocks, often in association with anhydrite, halite, celestine, native sulfur, sometimes with bitumen and oil. It is deposited in significant quantities by sedimentation in lake and sea salt-bearing dying basins. In this case, gypsum, along with NaCl, can be released only in the initial stages of evaporation, when the concentration of other dissolved salts is not yet high. When a certain value of salt concentration is reached, in particular NaCl and especially MgCl 2, anhydrite will crystallize instead of gypsum and then other, more soluble salts, i.e. The gypsum in these basins must belong to earlier chemical sediments. Indeed, in many salt deposits, layers of gypsum (as well as anhydrite), interbedded with layers of rock salt, are located in the lower parts of the deposits and in some cases are underlain only by chemically precipitated limestones.
In Russia, thick gypsum-bearing strata of Permian age are distributed throughout the Western Urals, in Bashkiria and Tatarstan, in Arkhangelsk, Vologda, Gorky and other regions. Numerous deposits of Upper Jurassic age are established in the North. Caucasus, Dagestan. Remarkable collection samples with gypsum crystals are known from the Gaurdak deposit (Turkmenistan) and other deposits in Central Asia (in Tajikistan and Uzbekistan), in the Middle Volga region, in the Jurassic clays of the Kaluga region. In the thermal caves of Naica Mine, (Mexico), druses of uniquely sized gypsum crystals up to 11 m long were found.
APPLICATION
 Today, the mineral "gypsum" is mainly a raw material for the production of α-gypsum and β-gypsum. β-gypsum (CaSO 4 ·0.5H 2 O) is a powdered binder material obtained by heat treatment of natural dihydrate gypsum CaSO 4 ·2H 2 O at a temperature of 150-180 degrees in apparatus communicating with the atmosphere. The product of grinding β-modification gypsum into a fine powder is called building gypsum or alabaster; with finer grinding, molding gypsum is obtained or, when using high-purity raw materials, medical gypsum.
Today, the mineral "gypsum" is mainly a raw material for the production of α-gypsum and β-gypsum. β-gypsum (CaSO 4 ·0.5H 2 O) is a powdered binder material obtained by heat treatment of natural dihydrate gypsum CaSO 4 ·2H 2 O at a temperature of 150-180 degrees in apparatus communicating with the atmosphere. The product of grinding β-modification gypsum into a fine powder is called building gypsum or alabaster; with finer grinding, molding gypsum is obtained or, when using high-purity raw materials, medical gypsum. During low-temperature (95-100 °C) heat treatment in hermetically sealed apparatus, α-modification gypsum is formed, the grinding product of which is called high-strength gypsum.
When mixed with water, α and β-gypsum hardens, turning back into gypsum dihydrate, with the release of heat and a slight increase in volume (by approximately 1%), however, such secondary gypsum stone already has a uniform fine-crystalline structure, the color of various shades of white (depending on raw materials), opaque and microporous. These properties of gypsum are used in various fields of human activity.
Gypsum (eng. Gypsum) - CaSO 4 * 2H 2 O
CLASSIFICATION
| Strunz (8th edition) | 6/C.22-20 |
| Nickel-Strunz (10th edition) | 7.CD.40 |
| Dana (7th edition) | 29.6.3.1 |
| Dana (8th edition) | 29.6.3.1 |
| Hey's CIM Ref. | 25.4.3 |
PHYSICAL PROPERTIES
| Mineral color | colorless turning to white, often colored by impurity minerals yellow, pink, red, brown, etc.; sometimes sectorial-zonal coloring or distribution of inclusions across growth zones inside crystals is observed; colorless in internal reflexes and at random. |
| Stroke color | white |
| Transparency | transparent, translucent, opaque |
| Shine | glassy, close to glassy, silky, pearlescent, dull |
| Cleavage | very perfect, easily obtained by (010), almost mica-like in some samples; along (100) clear, turning into a conchoidal fracture; according to (011), gives a splintered fracture (001) |
| Hardness (Mohs scale) | 2 |
| Kink | smooth, conchoidal |
| Strength | flexible |
| Density (measured) | 2.312 - 2.322 g/cm 3 |
| Radioactivity (GRapi) | 0 |
Class of sulfates, CaSO 4 .2H 2 O. In its pure form it contains 32.56% CaO, 46.51% SO 3 and 20.93% H 2 O. Mechanical impurities mainly in the form of organic and clay substances, sulfides, etc. Crystallizes into monoclinic. The crystal structure is based on double layers of anionic groups (SO 4) 2- linked by Ca 2+ cations. The crystals are tabular or prismatic, forming twins, the so-called dovetail. very perfect. Aggregates: granular, leafy, powdery, nodules, fibrous veins, radially needle-shaped. Pure gypsum is colorless and transparent, in the presence of impurities it has a gray, yellowish, pinkish, brown to black color. Glass shine. 1.5-2. 2300 kg/m3. B is noticeably soluble (2.05 g/l at 20°C). Mainly chemogenic in origin. Precipitates at a temperature of 63.5°C, and in solutions saturated with NaCl - at a temperature of 30°C. With a significant increase in salinity in drying sea lagoons and salt lakes, anhydrous calcium sulfate begins to precipitate instead of gypsum - similarly, anhydrite appears when gypsum dehydrates. Hydrothermal gypsum, which forms in low-temperature sulfide deposits, is also known. Varieties: - translucent fibrous aggregates, cast in reflected light with a beautiful silky sheen; gypsum spar - lamellar gypsum in the form of transparent crystals of a layered structure, etc.
Application of gypsum
Gypsum is used in raw and fired form. 50-52% of gypsum stone mined is used to produce gypsum binders for various purposes (GOST 195-79), obtained by firing natural gypsum, 44% of gypsum is used in the production of Portland cement, where gypsum is used as an additive (3-5%) to regulate timing setting of cement, as well as for the production of special cements: gypsum-alumina expanding cement, tensile cement, etc. 2.5% of gypsum is consumed by agriculture in the production of nitrogen fertilizers (ammonium sulfate) and for gypsuming saline soils; in non-ferrous metallurgy, gypsum is used as a flux, mainly in smelting; in paper production - as a filler, mainly in high grades of writing papers. In some countries (, etc.), gypsum is used for the production of sulfuric acid and cement. The ability of gypsum to be easily processed, to take polish well, and usually to have high decorative properties allows it to be used as a simulant in the production of facing slabs for the interior decoration of buildings and as a material for various crafts.
In the southern regions of the USSR, the national economy uses clay gypsum with a CaSO 4 .2H 2 O content of 40 to 90%. The loose rock consisting of gypsum and is called earthy gypsum, and in Transcaucasia and Central Asia it is called “drywall” or “ganch”. These rocks, in their raw form, are used for gypsuming soils, and in their calcined form, for plastering, as a binder.
Gypsum deposit
In the USSR, the largest deposits are located in the Tula, Kuibyshev, Perm regions of the RSFSR, in the Caucasus and Central Asia. In 150 deposits of gypsum and 22 deposits of clay-gypsum, drywall and ganch, reserves of 4.2 billion tons (1981) have been explored according to industrial categories. There are 11 deposits with gypsum reserves exceeding 50 million tons (including Novomoskovskoye - 857.4 million tons).
Gypsum is developed in quarries (Shedoksky, Saurieshsky plants, etc.) and mines (Novomoskovsky, Artyomovsky, Kamskoye Ustye, etc.). In the USSR, 42 deposits of gypsum and anhydrite and 6 deposits of gypsum-bearing rocks are exploited with an annual production of about 14 million tons (1981), of which 60.2% are in the territory
price
practicality
appearance
ease of manufacture
labor intensive to use
environmental friendliness
final grade
It is a sedimentary mineral found in nature in the form of layers of sedimentary rock. These are crystals of white, transparent color, having various shades from yellowish to red. The mineral is formed due to the evaporation of water saturated with calcium.
Today, gypsum is widely known as a building material used for finishing and plastering work. It is also used in architecture and landscape design.
Extraction, what gypsum is made from

Gypsum is mined in deposits by blasting gypsum-containing rock. The ore is then transported to factories in the form of gypsum stones. They are crushed in special crushers and then dried to evaporate the moisture present in them.
Dry fractions are crushed in mills to a powder state and sent to a kiln for firing. The powder is fired for 1-2 hours at a temperature of 150-160 degrees. The output is a finely dispersed white mixture, completely ready for use.
Place of Birth
Gypsum is widespread throughout Russia. The main places of gypsum production are the Vladimir, Arkhangelsk and Irkutsk regions, Central Asia, the Volga region, Bashkiria and the Western Urals. Other countries include Spain, Tunisia, Greece and Morocco.
Gypsum deposits arise due to the following factors:
- Weathering of salt deposits.
- In places where there are salt lakes, it forms as a chemical precipitate.
- It is an accompanying rock in old oil, sulfur and anhydrite deposits.
- Often mineral deposits are discovered at the mouths of ancient rivers.
The video shows how gypsum is mined and processed at the Forman plant:
Compound
The chemical composition is an aqueous solution of calcium sulfate. Its chemical formula is Ca? 2H2O. When heated to 140 degrees, water is released from its crystal lattice, resulting in the formation of so-called semi-aqueous gypsum.
If you continue heating the mineral, building (burnt) gypsum is formed. This material is used in powder form. If water is added again to such a powder, the water will join the calcium sulfate, and the material will become hard.
Sump
To separate gypsum and sand from the water mixture, special devices called gypsum separators are used. They allow you to collect gypsum and sand in a separate container, and direct the water to the drainage system. The sump must be connected between the sink and the drain pipe.
Additives for gypsum
Since gypsum consists of a rather fragile substance - calcium, to improve the quality of the resulting material, various substances and impurities are added to it.
Impregnations
Gypsum surfaces are porous and therefore require impregnation with special compounds. The pores are filled, and after drying the surface is considered ready for further painting. Natural drying oil is usually used as impregnation.
If it is not there, then you can use a solution of liquid glass or PVA glue. After applying the composition, you must wait for it to dry completely, and only then start painting the surface.
Plasticizer
With the help of additives such as plasticizers, it is possible to change and also control the degree of its fluidity. In addition, some types of plasticizers are capable of imparting additional strength to finished gypsum products. In general, there has been an increase in the production rate of gypsum products and more efficient and rational use of equipment.
Water repellent
Hydrophobizing compounds intended for introduction into gypsum solutions serve to reduce the water absorption of gypsum while maintaining its vapor permeability. This avoids the appearance of condensation on the surface of the plaster even when a sharp temperature difference occurs.
In addition, such additives increase the strength of the hardened product or gypsum surface and protect it from the formation of mold and mildew. The water repellent penetrates into the pores of the gypsum and begins to act immediately after drying.
The principle of operation of the water repellent

Varnish
Varnishes are used for finishing ready-made gypsum surfaces. The fact is that it is necessary to reduce the absorbency of gypsum, that is, to close its pores. To do this, it is recommended to saturate the surface with drying oil or varnish. It is better to use water-soluble varnishes based on acrylic or resin.
This composition penetrates deep into the pores of gypsum, and on its surface forms a thin and durable film with good adhesion. Such a surface will be reliably protected from moisture. For example, we can name several types of plaster varnish: “Izoplen”, “Dulux Trade Acrylic”, “Izo Sol”.
Glue
Some types of glue are used as additives in gypsum solutions. This not only increases the strength of the finished products, but also increases their water resistance. Most types of glue promote a slower setting of the solution. PVA glue, bone glue, wallpaper glue (WMC) and other types of adhesives are usually used.
Paint (pigment)
To create unusual colors, powdered iron oxide pigments are used. They are available in powder form in various colors. Pigments do not dissolve in water, organic solvents and other liquid media, so colored plaster will not lose its color over time.
Such pigments do not fade in the sun and do not change their color. Pigment powder is mixed with dry gypsum and distributed evenly throughout its entire volume.
Retarder
Gypsum tends to harden very quickly, so it is recommended to use retarders that can increase the durability of the gypsum mortar. The amount of such an additive depends on its type. Sodium tartrates, which are salts of tartaric acid, as well as sodium citrates (salts of citric acid) are used as additives.
In practice, it is more profitable to add ordinary citric acid to the water for the solution. In addition, PVA glue, liquid glass, animal glue, Dextrin or ready-made dry mixtures are also used as retarders.
Modifier
Polymer additives introduced into a gypsum solution can modify it, creating gypsum polymer composites. Depending on the amount of modifier introduced, the properties of the hardened product change.
With a minimum amount of them, strength increases and resistance to destruction in the open air increases. If you introduce a lot of modifier, the product will become waterproof, frost-resistant, and also increase its wear resistance.
Modifiers are usually produced in the form of a dry white powder. To use the modifier, the powder must be dissolved in water, which will be used to mix the gypsum.
How do modifiers affect the strength properties of gypsum mortars?

How to dissolve plaster
The dry gypsum mixture is dissolved with ordinary water. The higher the temperature of the water, the faster the reaction of the transition of the liquid solution into the solid state occurs. After hardening, gypsum does not dissolve in water.
However, if you store a hardened gypsum product or just a piece of hardened gypsum in conditions of high humidity, then the strength of the gypsum gradually decreases and the gypsum becomes brittle. To be reused, hardened gypsum is calcined in an oven to remove excess moisture and then ground into powder.
How to increase strength and make it stronger
To increase the strength of gypsum surfaces or products, it is recommended to introduce special additives into the solution. These are polymer fiber, various types of glue (CMC, PVA, bone glue), fluff lime, borax, liquid glass. Excellent results are obtained by reinforcing gypsum surfaces with a polymer mounting mesh.
To give a gypsum product strength comparable to the strength of ceramics, it is immersed for a day in a saturated solution of potassium alum. Then the product must be heated to a temperature of 550 degrees. You will be surprised by its durability.
How to make plaster at home
Gypsum is widely used in everyday life for the manufacture of all kinds of products. To prepare it at home, you need to prepare dry gypsum powder, water and dishes for stirring the composition. Pour water into the bowl, and then slowly pour the dry mixture into it, constantly stirring the solution.
Everything needs to be done carefully, but quickly, since the plaster can harden before anything is made from it. The entire cooking process should take no more than 2 minutes. It is recommended to use cold water.
The concentration of the solution should be as thick as liquid sour cream. If the solution turns out to be too liquid, add a little more dry mixture. But be careful, since you cannot add water to dilute an excessively dry solution.
The resulting mixture must be used as quickly as possible. If the solution has time to harden, then it is no longer acceptable to use it. Therefore, work with small portions of the gypsum mixture.
The video will tell you what is used as a plasticizer for gypsum when creating products from it with your own hands:
What is the average cost of this building material?
The cost of dry gypsum powder is quite affordable for the population. It depends on the packaging of the powder, the manufacturer and the cost of transport delivery to your region. The average price of 1 kilogram of building plaster ranges from 50 to 90 rubles. Medical plaster is more expensive. Its cost can reach up to 150 rubles per kilogram.